Answer:
Wavelength = 1.36 * 10^{-34} meters
Step-by-step explanation:
Given the following data;
Mass = 0.113 kg
Velocity = 43 m/s
To find the wavelength, we would use the De Broglie's wave equation.
Mathematically, it is given by the formula;
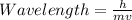
Where;
h represents Planck’s constant.
m represents the mass of the particle.
v represents the velocity of the particle.
We know that Planck’s constant = 6.6262 * 10^{-34} Js
Substituting into the formula, we have;
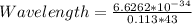
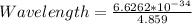
Wavelength = 1.36 * 10^{-34} meters