Answer:
-198 kJ
Step-by-step explanation:
Hello there!
In this case, as the reaction is not given but we can see that HBr is formed, we can infer that reaction is:
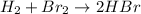
Whereas the -72 kJ per moles of HBr are:
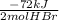
Thus, by multiplying by the formed 5.5 moles of HBr, the total energy release is:
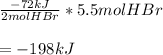
Best regards!