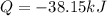Answer:
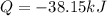
Step-by-step explanation:
From the question we are told that
Piston-cylinder initial Volume of air
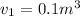
Piston-cylinder initial temperature
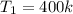
Piston-cylinder initial pressure
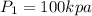
Supply line temperature
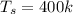
Supply line pressure
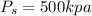
Valve final pressure
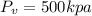
Piston movement pressure
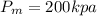
Piston-cylinder final Volume of air
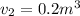
Piston-cylinder final temperature
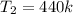
Piston-cylinder final pressure
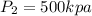
Generally the equation for conservation of mass is mathematically given by
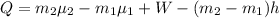
where
Initial moment
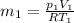
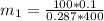
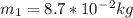
Final moment
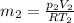
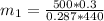
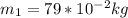
Work done by Piston movement pressure
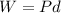
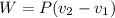
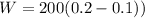
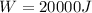
Heat function
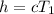
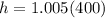
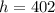
Therefore
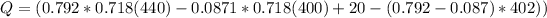
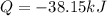
It is given mathematically that the system lost or dissipated Heat of