Answer:
V2 = 37.5 L
Step-by-step explanation:
Given the following data;
Initial volume, V1 = 25 L
Initial temperature, T1 = 20°C
Final temperature,T2 = 30°C
To find the final volume V2, we would use Charles' law;
Charles states that when the pressure of an ideal gas is kept constant, the volume of the gas is directly proportional to the absolute temperature of the gas.
Mathematically, Charles is given by;
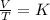
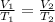
Making V2 as the subject formula, we have;
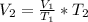
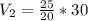
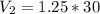
V2 = 37.5 L