Answer:
A) Not possible, B) Posible, C) Possible, D) Not possible.
Step-by-step explanation:
The maximum theoretical efficiency for any thermal engine is defined by Carnot's cycle, whose energy efficiency (
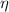 ), no unit, is expressed below:
), no unit, is expressed below:
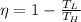 (1)
(1)
Where:
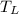 - Cold reservoir temperature, in Kelvin.
- Cold reservoir temperature, in Kelvin.
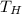 - Hot reservoir temperature, in Kelvin.
- Hot reservoir temperature, in Kelvin.
If we know that
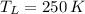 and
and
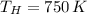 , then the maximum theoretical efficiency for the thermal engine is:
, then the maximum theoretical efficiency for the thermal engine is:
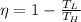
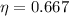
For real thermal engines, the following inequation is observed:
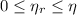 (2)
(2)
Where
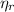 is the efficiency of the real heat engine, no unit.
is the efficiency of the real heat engine, no unit.
There are two possible criteria to determine if a given heat engine is real:
Efficiency
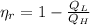 (3)
(3)
Where:
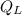 - Heat rejected to the cold reservoir, in kilojoules.
- Heat rejected to the cold reservoir, in kilojoules.
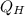 - Heat received from the hot reservoir, in kilojoules.
- Heat received from the hot reservoir, in kilojoules.
Power output
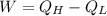 (4)
(4)
Where
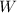 is the power output, in kilojoules.
is the power output, in kilojoules.
Now we proceed to verify each case:
A)
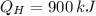 ,
,
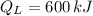 ,
,
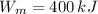
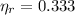
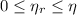
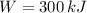
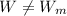
This engine is not possible.
B)
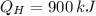 ,
,
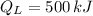 ,
,
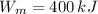
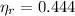
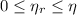
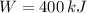
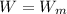
The engine is possible.
C)
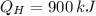 ,
,
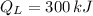 ,
,
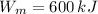
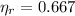
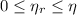
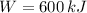
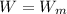
The engine is possible.
D)
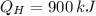 ,
,
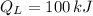 ,
,
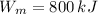
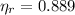
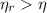
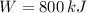
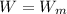
The engine is possible.