Answer:
mi = 31.28 g
Step-by-step explanation:
According to the law of conservation of energy:
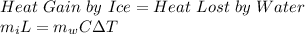
where,
mi = mass of ice = ?
L = Latent heat of fusion of ice = 334 J/g
mw = mass of water = 50 g
C = specific heat of water = 4.18 J/g.°C
ΔT = change in temperature of water = 75°C - 25°C = 50°C
Therefore,
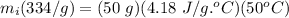
mi = 31.28 g