Answer:
The correct option is (a).
Step-by-step explanation:
One Atomic mass unit is the mass of an atom. It is defined as the mass of a single carbon -12 at rest.
The relation between amu and kg is given by :
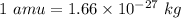
Hence, one amu is equal to the mass of a single atom of carbon.