Answer:
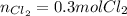
Step-by-step explanation:
Hello there!
In this case, according to the given chemical reaction whereas the sodium chloride is in a 2:1 mole ratio with chlorine, the required moles of the later are computed as shown below:
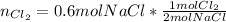
So we cancel out the moles of NaCl to obtain:
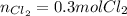
Best regards!