Answer:
8.33 L .
Step-by-step explanation:
Hello there!
In this case, according to the given problem, it is possible to firstly write up the undergoing chemical reaction:
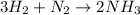
Whereas the mole ratio of hydrogen to nitrogen is 3:1. In such a way, given that the mole ratios can be also applied in volume when the temperature and pressure remain unchanged, it is possible to compute the required volume of nitrogen from 25.0 L of hydrogen as shown below:
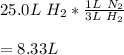
Best regards!