Answer:

Step-by-step explanation:
Since we are given the mass, specific heat, and change in temperature, we should use this formula for heat:
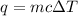
The substance's mass is 450.0 grams, the specific heat is 1.264 J/g°C, and the change in temperature is 7.1 °C.
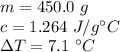
Substitute the values into the formula.
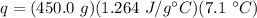
Multiply the first 2 values together. The grams will cancel out.
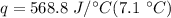
Multiply again. This time, the degrees Celsius cancel out.
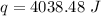
4038.48 Joules of heat energy are released.