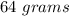Answer:
The amount of HI is "64 grams".
Step-by-step explanation:
The given values are:
Volume,
= 0.500 L
Molarity,
= 1.00 H
Molar mass of HI,
= 128
Now,
The moles of HI will be:
=
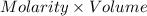
On substituting the values, we get
=
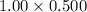
=
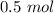
hence,
The amount of HI will be:
=
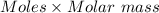
=
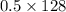
=