Answer:
A)
4, 7, 4, 6
B)
12 moles
Step-by-step explanation:
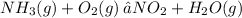
__↑______↑
8.00 mol | 14.00 mol
________________
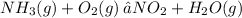
You can turn this into a system of variables which are solvable.
To do this, create variables for the coefficients of each compound in the reaction respectively.
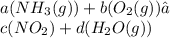
Because to be balanced, the count of atoms in each element of the compound correspond to the coefficient of the variable in that compound so that the count of the left (reactant) side is set equal to the right (product) side.
a corresponds to the coefficient of the first compound, b corresponds to the coefficient of the second compound, c corresponds to the coefficient of the third compound, and d corresponds to the coefficient of the fourth compound.
(Reactant = Product)
Reactant: 1a [N] Product: 1c.
Reactant: 3a [H] Product: 2d.
Reactant: 2b [O] Product: 2c + 1d.
Thus the system is:
1a = 1c
3a = 2d
2b = 2c + 1d.
Then just use the substitution methods to solve.