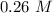Answer:
The molarity will be "0.26 M".
Step-by-step explanation:
The given values are:
Mass,
= 2.00 g
Volume of solution,
= 100 mL
or,
=
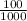
=
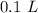
Molar mass of KCl,
= 74.5 gm
Now,
Moles of KCl will be:
=
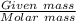
=
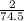
=
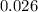
hence,
The molarity will be:
=
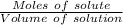
On substituting the values, we get
=
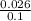
=