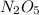Answer:
Step-by-step explanation:
usually, the first step is to calculate the moles but since that is already given in the question we can move onto the next step
least molar ratio:
N =
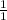 = 1
= 1
O =
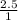 = 2.5
= 2.5
when the answer is in decimals we will multiply it by an integer in order to make it a whole number (in this case 2)
O = 2.5(2) = 5
in order to balance, we will also multiply the atoms of N by the same integer
N = 1(2) = 2
thus, the empirical formula is