Answer:
Volume of 6.5 moles of Helium at STP is 145.6954 L
Step-by-step explanation:
As we know
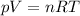
At STP, temperature is 273.15 K and pressure is 1 atm
n is the number of moles which is equal to 6.5
R is the gas constant = 0.08206 L atm/K mol
Substituting the given values in above equation, we get -
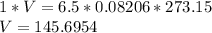 Liters
Liters
Volume of 6.5 moles of Helium at STP is 145.6954 L