Answer:
h = 1.01 x 10⁻³⁴ J.s
Step-by-step explanation:
The energy applied by the voltage must be equal to the energy associated with the wavelength of light:
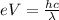
where,
e = charge on electron = 1.6 x 10⁻¹⁹ C
V = stopping potential
h = Planck's Constant = ?
c = speed of light = 3 x 10⁸ m/s
λ = wavelength of light
For λ = 400 nm = 4 x 10⁻⁷ m, V = 0.7 V:
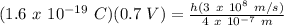
h = 1.49 x 10⁻³⁴ J.s
For λ = 500 nm = 5 x 10⁻⁷ m, V = 0.2 V:
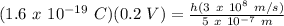
h = 0.53 x 10⁻³⁴ J.s
Taking average of both values:
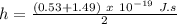
h = 1.01 x 10⁻³⁴ J.s