Answer:
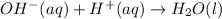
Step-by-step explanation:
Hello there!
In this case, according to the given reactants, it is firstly necessary to set up the complete molecular reaction:
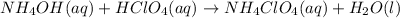
Now, by knowing that ionic species are able to ionize, we are able to write the complete ionic equation:
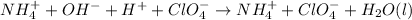
Whereas ammonium and chlorate ions are spectator ions because they remain the same on both sides of the equation. Therefore the total or net ionic equation would be:
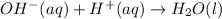
Best regards!