Answer:
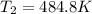
Step-by-step explanation:
Hello there!
In this case, according to the the variable temperature and pressure and constant volume, it turns out possible for us to calculate the new temperature via the Gay-Lussac's law as shown below:
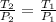
Thus, by solving for the final temperature, T2, we obtain:
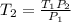
So we plug in the given data to obtain:
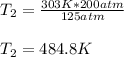
Best regards!