Answer:
P = 13.5 atm
Step-by-step explanation:
Given that
No. of moles, n = 20 moles
Volume of nitrogen gas = 36.2 L
Temperature = 25°C = 298 K
We need to find the pressure of the gas. Using the ideal gas equation
PV = nRT
Where
R is gas constant,
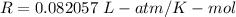
So,
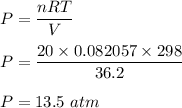
so, the pressure of the gas is equal to 13.5 atm.