Answer:
m = 49.8 g
Step-by-step explanation:
Hello there!
In this case, for this calorimetry processes we can define the involved heat in terms of mass, specific heat and temperature as shown below:
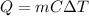
Thus, given the heat, final and initial temperature and specific heat of water (4.184), the mass of water can be computed as shown below:
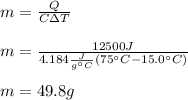
Best regards!