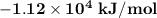Answer:
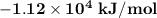
Step-by-step explanation:
Given that:
mass (m) of the sample = 1.02 g
number of moles of stearic acid
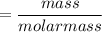
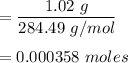
The change in temp.
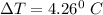
heat capacity of the calorimeter (c) = 9.43 kJ/° C
Thus, heat due to reaction = cΔT
= 9.43 kJ/° C × 4.26° C
= 40.17 kJ
The heat in kJ/mol =
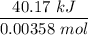
= 11204.23 kJ/mol
= 1.12 × 10⁴ kJ/mol
As a result of the reaction is exothermic, the heat reaction of the combustion is: