Answer: a. A solid melts:
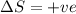
b. a vapor is converted to solid:
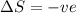
c. a liquid freezes:
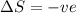
d. A solid sublimes:
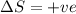
e. a vapor condenses to liquid :
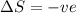
f. a liquid boils:
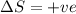
g. dissolving a tablespoon of salt in water:
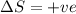
h. combustion of gasoline:
Step-by-step explanation:
Entropy is the measure of randomness or disorder of a system. If a system moves from an ordered arrangement to a disordered arrangement, the entropy change is negative and vice versa.
a. A solid melts: The solid is converting to liquid, thus the randomness is increasing as the molecules are moving away and the intermolecular forces are getting weaker. Thus the entropy change
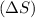 is positive.
is positive.
b. a vapor is converted to solid: The gas is converting to solid, thus the randomness is decreasing as the molecules are moving close and the intermolecular forces are getting stronger Thus the entropy change
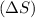 is negative.
is negative.
c. a liquid freezes: The liquid is converting to solid, thus the randomness is decreasing as the molecules are moving close and the intermolecular forces are getting stronger. Thus the entropy change
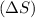 is negative.
is negative.
d. A solid sublimes: The solid is converting to gas, thus the randomness is increasing as the molecules are moving away and the intermolecular forces are getting weaker. Thus the entropy change
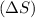 is positive.
is positive.
e. a vapor condenses to liquid : The gas is converting to liquid, thus the randomness is decreasing as the molecules are moving close and the intermolecular forces are getting stronger. Thus the entropy change
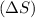 is negative.
is negative.
f. a liquid boils: The liquid is converting to gas, thus the randomness is increasing as the molecules are moving away and the intermolecular forces are getting weaker. Thus the entropy change
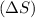 is positive.
is positive.
g. dissolving a tablespoon of salt in water: The solid is converting to ions , thus the randomness is increasing as the ions can move freely. Thus the entropy change
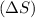 is positive.
is positive.
h. combustion of gasoline: The liquid is converting to gas, thus the randomness is increasing as the molecules are moving away and the intermolecular forces are getting weaker. Thus the entropy change
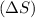 is positive.
is positive.