Answer:
Energy released = 18.985 J
Step-by-step explanation:
The exponential decay of radioactive substance is given by -
N(t) = N₀
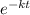
where
N₀ = initial quantity
k = decay constant
Half life,
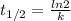
⇒
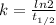
Given,
N₀ = 12.5 + 3 = 15.5 × 10⁻⁶ gm
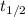 = 32.6 + 18 = 50.6 × 10⁶ years
= 32.6 + 18 = 50.6 × 10⁶ years
So,
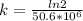 = 1.361 × 10⁻⁸ year⁻¹
= 1.361 × 10⁻⁸ year⁻¹
Now,
N(1) = 15.5 × 10⁻⁶
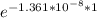
= 15.49999978904
Now,
Substance decayed = N₀ - N(t)
= 15.5 × 10⁻⁶ - 15.49999978904 × 10⁻⁶
= 21.095 × 10⁻¹⁷ kg
⇒Δm = 21.095 × 10⁻¹⁷ kg
So,
Energy released = Δmc²
= 21.095 × 10⁻¹⁷ × 3 ×10⁸ × 3 × 10⁸
= 189.855 ×10⁻¹
= 18.985 J
⇒Energy released = 18.985 J