Answer:
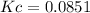
Step-by-step explanation:
Hello there!
In this case, according to the given chemical reaction:
CaCO3(s)→CaO(s)+CO2(g)
In which the amounts are unfortunately given, we can however, assume the information of similar problems so you can further modify the numbers but follow the same work:
CaCO3 = 25.3 g
CaO = 14.9 g
CO2 = 33.7 g
Thus, since just CO2 is involved on the equilibrium expression because CaCO3 and CaO are solid, we can compute the moles of the CO2 at equilibrium and further compute the concentration in the 9.0-L vessel:
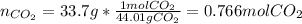
![[CO_2]=(0.766molCO_2)/(9.0L)=0.0851M](https://img.qammunity.org/2022/formulas/chemistry/college/ve7ziw07px72xk826ub8211jbvvomf8d4y.png)
Thus, we proceed with the equilibrium expression to obtain:
![Kc=[CO_2]\\\\Kc=0.0851](https://img.qammunity.org/2022/formulas/chemistry/college/a164p5sy68yyh1pvvjpqmit7obxa1hjmfa.png)
Best regards!