Answer:
The specific heat of iron is 0.44
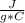 .
.
Step-by-step explanation:
Calorimetry is responsible for measuring the amount of heat generated or lost in certain physical or chemical processes.
There is a direct proportionality relationship between the temperature (Two quantities are directly proportional when there is a constant so that when one of the quantities increases, the other also increases; and the same happens when either of the two decreases.). The constant of proportionality depends on the substance that constitutes the body and its mass, and is the product of the specific heat and the mass of the body.
The amount of heat (Q) necessary to vary the temperature of a mass (m) of a substance is proportional to the change in its temperature (∆T) and to that mass, that is:
Q = c * m * ΔT
where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation.
In this case:
- Q= 1000 J
- c= ?
- m= 22.5 g
- ΔT= Tfinal - Tinitial= 125 C - 25 C= 100 C
Replacing:
1000 J= c*22.5 g* 100 C
Solving:
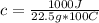
c= 0.44
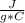
The specific heat of iron is 0.44
 .
.