Answer:
Mass of sodium metal is 130.87 gram
Step-by-step explanation:
The complete reaction is
2 NaN3 --> 2 Na + 3 N2
We know that PV = nRT
n =
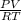
On substituting the given values, we get
 moles of N2
moles of N2
Sodium azide's molar mass
3.02 *(2/3) = 2.013 moles
Mass = 2.03 * 65.01 = 130.87 gram