Answer:
626.3K or 353.15°C
Step-by-step explanation:
We will be using the Combined Gas Law for this problem. The Combined Gas Law (as you guessed it!) combines Boyle's Law, Charles' Law, and Gay-Lussac's Law into a single equation for situations where P, V, and T all vary. It expresses the relationship between pressure, volume, and temperature of a fixed amount of gas. Remember, it allows calculations where only the amount of gas is constant.
Note: When using this law, temperature (T) MUST be in Kelvin Temperature. I can't stress how important it is to convert the temperature value into Kelvin degrees because forgetting to do so will mess up your answer.
Combined Gas Law:
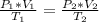
In this problem, all of the values for the left side of the equation are given: P1 = 0.5 atm, V1 = 0.50L, and T1 = 40°C + 273.15 = 313.15K. For the right side of the equation, we are given the volume and pressure of the expanded gas and are told to find the new temperature (T2): P2 = 0.10 atm and V2 = 5.0L. Because the pressure and volume for both sides of the equations are provided in the same unit, there is no need for conversions.
Now onto the math - all that is needed to be done is plugging in the values and solving for T2. The work is shown below:
Set up:
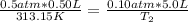
==>
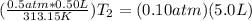
==>
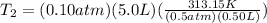
==>
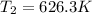
At this point, you can leave your answer as 626.3K. However, because the temperature of the original gas was given in Celsius, I would recommend converting it back to that provided unit. So, our final step is: 626.3K - 273.15 = 353.15°C.