Answer:
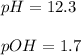
![[H^+]=5x10^(-13)M](https://img.qammunity.org/2022/formulas/chemistry/high-school/m7pxuu6tl7san18cwsz0q1z90anb6269qg.png)
![[OH^-]=0.02M](https://img.qammunity.org/2022/formulas/chemistry/high-school/5z92953qxtr09vc3akco21k7399qv67hzf.png)
Step-by-step explanation:
Hello there!
In this case, according to the given ionization of magnesium hydroxide, it is possible for us to set up the following reaction:
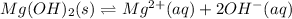
Thus, since the ionization occurs at an extent of 1/3, we can set up the following relationship:
![(1)/(3) =(x)/([Mg(OH)_2])](https://img.qammunity.org/2022/formulas/chemistry/high-school/4kl4qegetojfuvoygylgo8ax4k9gzkn44e.png)
Thus, x for this problem is:
![x=([Mg(OH)_2])/(3)=(0.03M)/(3)\\\\x= 0.01M](https://img.qammunity.org/2022/formulas/chemistry/high-school/tq2en7n72280x2zlbr8sg9cjwihn99z749.png)
Now, according to an ICE table, we have that:
![[OH^-]=2x=2*0.01M=0.02M](https://img.qammunity.org/2022/formulas/chemistry/high-school/2jm1nhq5j8kk8f4vgsnjsk4j1vws1fr46u.png)
Therefore, we can calculate the H^+, pH and pOH now:
![[H^+]=(1x10^(-14))/(0.02)=5x10^(-13)M](https://img.qammunity.org/2022/formulas/chemistry/high-school/ddu4zan8tzcuqb5zn75vf2i8cyuxlgre12.png)
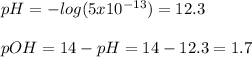
Best regards!