Answer:
221.22K or -51°C
Step-by-step explanation:
We will be using the Ideal Gas Law to calculate the temperature of the gas. It is a mathematical relationship that describes the behavior of ideal gas ample for any combo of varying pressure, volume, temperature, and # of moles (n). It is derived by combing Boyle's Law, Charles' Law, Gay-Lussac's & Avogadro's Law.
Note: As always, remember that temperature must be in Kelvin not Celsius when using this equation.
Ideal Gas Law:
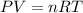 , where P = pressure, V = volume (in Liters), n = # of moles, R = the ideal gas constant, and T = temperature (in Kelvin).
, where P = pressure, V = volume (in Liters), n = # of moles, R = the ideal gas constant, and T = temperature (in Kelvin).
Based on the problem, we are given the pressure, volume, and # of moles. We are asked to find the temperature. What about R you ask? Well, R is a constant that is the value of 1 mole of gas at STP. R has various values depending on the pressure units. In this case, our pressure is in atm so the R value = 0.0821.
Onto the math - all that needs to be done now is to plug and chug. Plug in the given values to find the temperature:
Set up:
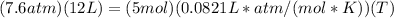
==>
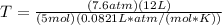
==> T = 221.17K
The answer is 221.17K. To convert into Celsius, subtract by 273.15 to get -50.99 or -51°C.