Answer:
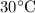
Step-by-step explanation:
To know the temperature at which KCl dissolves in water we need to refer to the general solubility curves.
In the case of
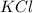 ,
,
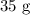 of it will dissolve in
of it will dissolve in
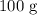 of water at a minimum temperature of
of water at a minimum temperature of
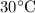 .
.
So, the the minimum temperature needed to dissolve 35 grams of KCl in 100 grams of water is
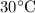 .
.