Answer:
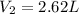
Step-by-step explanation:
Hello there!
In this case, according to the given problem, it is possible for us to notice that the equation to be used is the Avogadro's one in terms of mass because this is applied to the same gas, hydrogen:
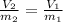
Thus, by considering both the initial and final masses and the initial volume, we can compute the final volume as shown below:
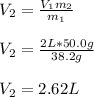
Best regards!