Answer:
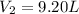
Step-by-step explanation:
Hello there!
In this case, according to the given STP (standard pressure and temperature), it is possible for us to realize that the equation to use here is the Avogadro's law as a directly proportional relationship between moles and volume:
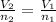
In such a way, given the initial volume and both initial and final moles, we can easily compute the final volume as shown below:
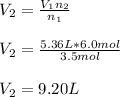
Best regards!