Answer:
292.75mL
Explanation:
From the ideal gas equation
PV= nRT
With each symbols having standard meaning
For pressure remaining constant the equation for two states can be written as
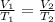
Given V_1 = 300mL, T_1 = 17° C = 290K and T_2 = 10°C or 283K
Now plugging values in above equation to find V_2
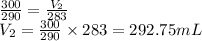
Therefore, the volume at 10 degrees Celsius = 292.75mL