Answer:
H = 226,311.66J
Step-by-step explanation:
Hello there!
In this case, according to the thermodynamic path ice takes up to steam:
1. Ice (-15°C) --> Ice (0°C) H1
2. Ice (0°C) ---> Water (0°C) H2
3. Water (0°C) --> Water (100°C) H3
4. Water (100°C) --> Steam (100°C) H4
5. Steam (100°C) --> Steam (145°C) H5
It is possible for us to infer that H1, H3 and H5 are computed by means of the mass, specific heat and change in temperature as sensible heat. On the flip side, H2 and H4 are computed as latent heat. Thus, we proceed as follows:
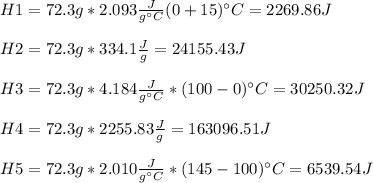
Then, we add them up to obtain the total heat:
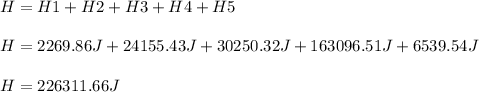
Best regards!