Answer:
![[C]=0.0221M](https://img.qammunity.org/2022/formulas/chemistry/high-school/1a18nsbtvvsptpx196kwyvqn1qy2eu2o2w.png)
Step-by-step explanation:
Hello there!
In this case, according to the given chemical reaction at equilibrium:
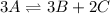
It is possible for us to set up the equilibrium expression as shown below:
![Kc=([B]^3[C]^2)/([A]^3)](https://img.qammunity.org/2022/formulas/chemistry/high-school/hgqjyoa9jmppzpxtz8o2ywt0sce5raezpf.png)
Whereas Kc, [A] and [B] are known as 0.0091, 0.031M and 0.0823M and it is required to calculate [C]; thus, we solve for it as follows:
![[C]^2=([A]^3Kc)/(B]^3)](https://img.qammunity.org/2022/formulas/chemistry/high-school/2mrly63aovuw1max1ian0lua5yy13e2i9m.png)
![[C]=\sqrt{((0.031)^3*0.0091)/((0.0823)^3)}](https://img.qammunity.org/2022/formulas/chemistry/high-school/bfknugkuqibl2icvkeg31slhl7h81jgddv.png)
![[C]=0.0221M](https://img.qammunity.org/2022/formulas/chemistry/high-school/1a18nsbtvvsptpx196kwyvqn1qy2eu2o2w.png)
Best regards!