Answer:
0.320 g Cu.
Step-by-step explanation:
Hello there!
In this case, case, according to the presented chemical reaction, it is possible to determine that the mole ratio of zinc to copper is 1:1 and therefore, we will have the same amount of atoms for the both of them. Moreover, since one mole of copper contains 6.022x10²³ atoms of this element, with a mass of 63.546 g (molar mass); it is possible for us to write up the following mathematical setup in order to calculate the produced grams of copper:
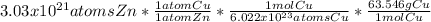
Thus, the numerical result turns out to be:
0.320 g Cu
Best regards!