Answer:
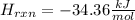
Step-by-step explanation:
Hello there!
In this case, according to the given information, it is possible to realize that the heat released by the reaction is used up to heat up the mixture, which means we can write:
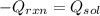
Whereas the Q of solution is given by the total mass (3.644+42.054), the specific heat of the solution and the change in temperature; thus, we obtain:
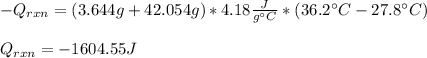
Next, by computing the moles of the solute, we can proceed to compute the kJ/mol for the enthalpy of reaction (dissolution):
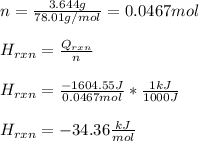
Best regards!