Answer:
280 liters at STP are in 8.0*10² grams of sulfur dioxide.
Step-by-step explanation:
The STP conditions refer to the standard temperature and pressure. Pressure values at 1 atmosphere and temperature at 0 ° C are used and are reference values for gases. And in these conditions 1 mole of any gas occupies an approximate volume of 22.4 liters.
In this case, the molar mass of sulfur dioxide, that is, the mass of one mole of the substance, is 64 g/mole.
You can apply the following rule of three: if by definition of molar mass 64 grams of sulfur dioxide are contained in 1 mole, 8.0*10² grams are contained in how many moles?
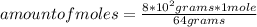
amount of moles= 12.5 moles
Now you can apply a new rule of three: if by STP conditions 1 mole of any gas occupies a volume of 22.4 liters, 12.5 moles will occupy how much volume?
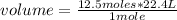
volume= 280 L
280 liters at STP are in 8.0*10² grams of sulfur dioxide.