Answer:
0.04 L (or 40 mL)
Step-by-step explanation:
The dilution equation is:
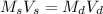
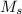 = the molarity of the sock solution
= the molarity of the sock solution
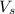 = the volume of the sock solution
= the volume of the sock solution
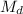 = the molarity of the diluted solution
= the molarity of the diluted solution
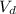 = the volume of the diluted solution
= the volume of the diluted solution
We are given the original, or stock, solution, which is
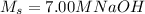 , and
, and
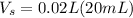 . We are also given the final molarity, which is:
. We are also given the final molarity, which is:
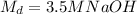 .
.
So, plugging our given into the dilution equation, results in:
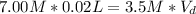 (divide both sides by 3.5 M, in order to get
(divide both sides by 3.5 M, in order to get
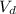 by itself).
by itself).
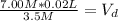
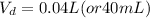
So, the final volume of a 3.5 M NaOH solution, with an original solution of 20 mL of a 7.00 M NaOH solution, is 0.04 L (or 40 mL)
Hopefully this helped. Good luck!