Answer:
1.62x10⁻¹² M
Step-by-step explanation:
HClO₃ is a strong acid, meaning that a 6.2x10⁻³ M solution has a molar concentration of H⁺ of 6.2x10⁻³.
With the above information in mind, we can calculate the pH of the solution:
- pH = -log(6.2x10⁻³) = 2.21
Now we can calculate the pOH of the solution, using the pH:
Finally we calculate [OH⁻], using the definition of pOH:
- [OH⁻] =
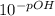 = 1.62x10⁻¹² M
= 1.62x10⁻¹² M