Answer:
Step-by-step explanation:
To determine the polarity of a bond, we use the electronegativities of the individual atoms. The greater the difference, the more polar the bond.
1. H-Br
- Hydrogen: 2.2
- Bromine: 3.0
3.0-2.2=0.8
2. H-F
- Hydrogen: 2.2
- Fluorine: 4.0
4.0-2.2=1.8
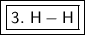
3. H-H
2.2-2.2=0.0
4. H-O
3.4-2.2=1.2
As we can see, the lowest difference is in hydrogen hydrogen at 0.0. You may also recognize that because they are 2 atoms of the same element, the electronegativity is the same for both, so it will equal 0. Since the electronegativity is 0, this is a nonpolar bond.