To answer this question, we need to first understand the concepts of Volume and Density. Volume is a pretty easy concept to grasp - it is the overall space of a three-dimensional object. Density is essentially a rate, mass per unit of volume.
For example, let's say we have an object that has a volume of 50
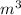 (cubic meters). If this object has a density of 10
(cubic meters). If this object has a density of 10
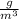 (grams per cubic meter), that essentially means that the object weighs 10 grams for every cubic meter of space it occupies. Because the object occupies 50 cubic meters of space, we know that the total weight will ultimately be 500 grams, as 50 times 10 is 500.
(grams per cubic meter), that essentially means that the object weighs 10 grams for every cubic meter of space it occupies. Because the object occupies 50 cubic meters of space, we know that the total weight will ultimately be 500 grams, as 50 times 10 is 500.
Okay, so we have an example. What can we do with it? Let's try to make an equation out of this example we have. We can see that the total weight of the object is found by multiplying density and volume. Let's write that in an equation:
m = d * V
in which:
m - mass
d - density
V - volume
This equation makes total sense, too! Density is essentially
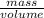 , and by multiplying it by volume it cancels out the volume, leaving behind only the final mass!
, and by multiplying it by volume it cancels out the volume, leaving behind only the final mass!
You may be thinking ,"well, this equation solves for mass, not volume. We're trying to solve for volume, aren't we?" You're right! Let's isolate V in order to create our final equation:
m = d * V
Divide both sides by d:
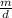 =
=
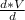
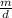 = V
= V
V =
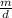
Awesome! We have our equation! Let's get the numbers we were given from the equation and plug them in:
V =
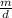
V =
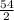
V = 27
And that's our answer! If you need any further explanation, just let me know!
- breezyツ