Answer:
Because of the electronegativity difference C-O (covalent) and Ca-O (ionic) bonds have.
Step-by-step explanation:
Hello there!
In this case, in order to understand the polarity of this compound, calcium carbonate, it firstly necessary that it is comes from the definition of electronegativity, as a property defining the capacity to attract electrons from another atom; thus, since the electronegativities of calcium, carbon an oxygen are 1.0, 2.55 and 3.44, it is possible to realize this compound has O-Ca and O-C bonds according to its Lewis dot structure. In such a way, we calculate the difference electronegativity for these two bonds:
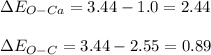
Thus, since 2.44 accounts for an ionic bond and 0.89 for a covalent one, we bear out the given statement in the question.