Answer:
The difference in the amounts of carbon powder and ammonium carbonate is
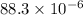 grams.
grams.
Explanation:
According to this statement, we have a mixture with
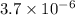 grams of carbon powder and
grams of carbon powder and
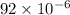 grams of ammonium carbonate. The difference between those amounts consist in subtracting the latter quantity for the former one. That is:
grams of ammonium carbonate. The difference between those amounts consist in subtracting the latter quantity for the former one. That is:
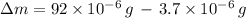
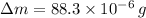
The difference in the amounts of carbon powder and ammonium carbonate is
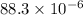 grams.
grams.