Answer: The percent by mass concentration is 33.3 %
Step-by-step explanation:
Mass percent is the ratio of mass of solute to the mass of solution in terms of percentage.
mass of solute = 50 g
mass of solution =
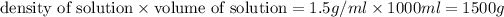
Mass percentage =
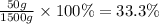
Thus percent by mass concentration of 1000 ml of a solution (d=1.5 g/ml) that contains 50 g of solute in it is 33.3 %