Answer: The pH of the solution is 8.8
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.pOH is calculated by taking negative logarithm of hydroxide ion concentration
![pH=-\log [H^+]](https://img.qammunity.org/2022/formulas/chemistry/college/d4u8c7rky5aqengst85apsbxbk128yl4er.png)
![pOH=-log[OH^-]](https://img.qammunity.org/2022/formulas/chemistry/high-school/xdplb04m9cfjwuofncjx7my18bccdxxye9.png)
pH+pOH=14
Putting in the values:
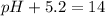
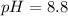
Thus pH of the solution is 8.8