Answer:
1. The balanced chemical reaction is:
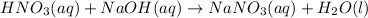
2. The balanced chemical reaction is:
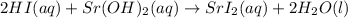
Step-by-step explanation:
Hello there!
In this case, according to the given chemical reactions, it is necessary to keep in mind the cations and anions are switched around as a result of the bonds rearrangement in these neutralization reactions; therefore, we can proceed as follows:
1. The balanced chemical reaction is:
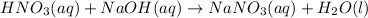
Because all atoms are in the same amount at each side of the equation and the resulting salt is soluble.
2. The balanced chemical reaction is:
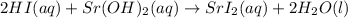
Because all atoms are in the same amount at each side of the equation and the resulting salt is soluble.
Best regards!