Answer:
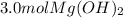
Step-by-step explanation:
Hello there!
In this case, for these types of acid-base neutralizations, it is crucial to firstly set up the chemical reaction taking place between the acid and the base; in this case HCl and Mg(OH)2 respectively, whose products are obtained by switching around the anions and cations as shown below:
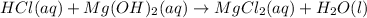
Which must be balanced to accurately predict the mole ratio on the reactants side:
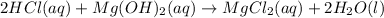
Whereas we can see a 2:1 mole ratio of the acid to the base; thus, the moles of Mg(OH) required for the neutralization of 6.0 moles of HCl turn out to be:
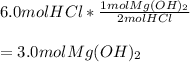
Best regards!