Answer:

Step-by-step explanation:
To find the mole ratio, we must use the coefficients.
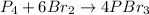
This equation is balanced (4 atoms of P and 12 atoms of Br on both sides), so we can go straight to the coefficients.
- P₄ doesn't have a coefficient, so 1 is implied
- Br₂ has a coefficient of 6
- PBr₃ has a coefficient of 4
So according to the coefficients above, for every 1 mole of P₄ that is consumed, 4 moles of PBr₃ are produced. Therefore, the ratio is 1 mole P₄ to 4 moles of PBr₃.