Answer:
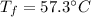
Step-by-step explanation:
Hello there!
In this case, for this calorimetry problem, it is possible to realize that the hot water at 87.6 °C is cooled down whereas the cold water at 34.5 °C is heated up, according to:
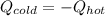
Which in terms of mass, specific heat (cancelled out because they have the same value for being water) and temperature difference, is:
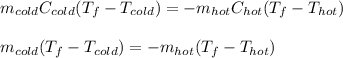
Thus, solving for the final temperature, we obtain:
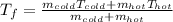
Then, we plug in to obtain:
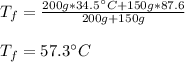
Best regards!